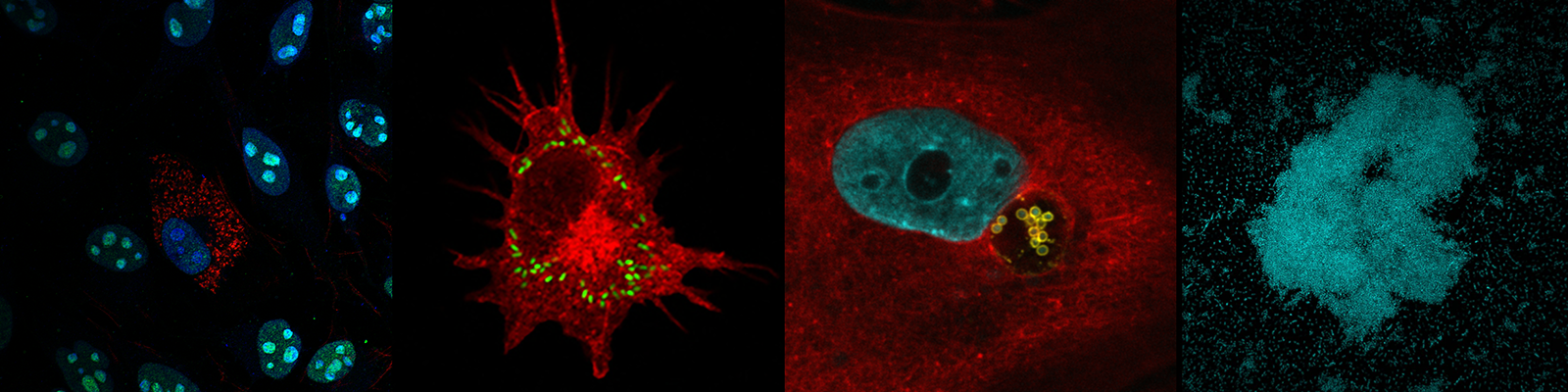

Our team studies how bacteria subvert host responses to cause disease. Our long-term goal is to unravel the molecular and cellular basis of the infectious process to develop new approaches to prevent the dissemination and virulence of these bacteria.

Our favorite pathogens
Brucella abortus
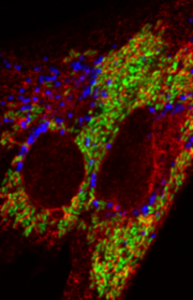
Brucella causes one of the most prevalent zoonoses worldwide, still endemic in many developing countries. Brucellosis can have a severe economic impact, affecting a wide range of animals, including ruminants, swine, and dogs. The presence of brucellosis in marine and terrestrial wildlife is also of concern. Brucella can invade and extensively replicate inside host cells by injecting specific proteins that tightly control cellular functions. Despite extensive intracellular replication, Brucella species have developed exquisite mechanisms to induce or inhibit inflammation depending on the tissue context, modulate cell signaling, and interfere with host metabolism. These proteins are the focus of our research.
Acinetobacter baumannii
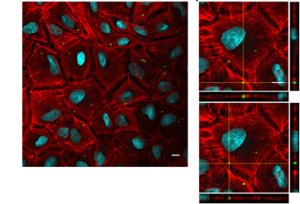
Infections with multi-drug resistant bacteria are a leading cause of morbidity and mortality worldwide. A. baumannii is rapidly becoming a global health threat. It has a remarkable ability to persist in the environment and to acquire multi-drug resistance and virulence traits, enhancing its ability to cause life-threatening infections, particularly in intensive care units. Recent work suggests animals may present an important reservoir for the bacteria, potentially increasing antibiotic resistance spread. Characterizing A. baumannii-host interplay, considering its human-animal-environment context, is a central topic of our lab’s research.
News flash
January 2026
New lab space!!!!!!!!!! The Salcedo lab has moved to the new Vet Med building
December 2025
Charline’s first paper is out in Microbiology!
In collaboration with the Kroger lab at Trinity College we found that the Tat export pathway contributes to adhesion of A. baumannii ABC141 strain to endothelial and epithelial cells.
July 2025
Congratulations Dr. Charline Debruyne and Dr. Lison Cancade-Veyre for obtaining their PhDs after two excellent Viva exams!
June 2025
Publication of Lison’s first article as a first author! Very nice little story showing the important role of dimerization for the function of the Brucella abortus effectors NyxA and NyxB during infection.
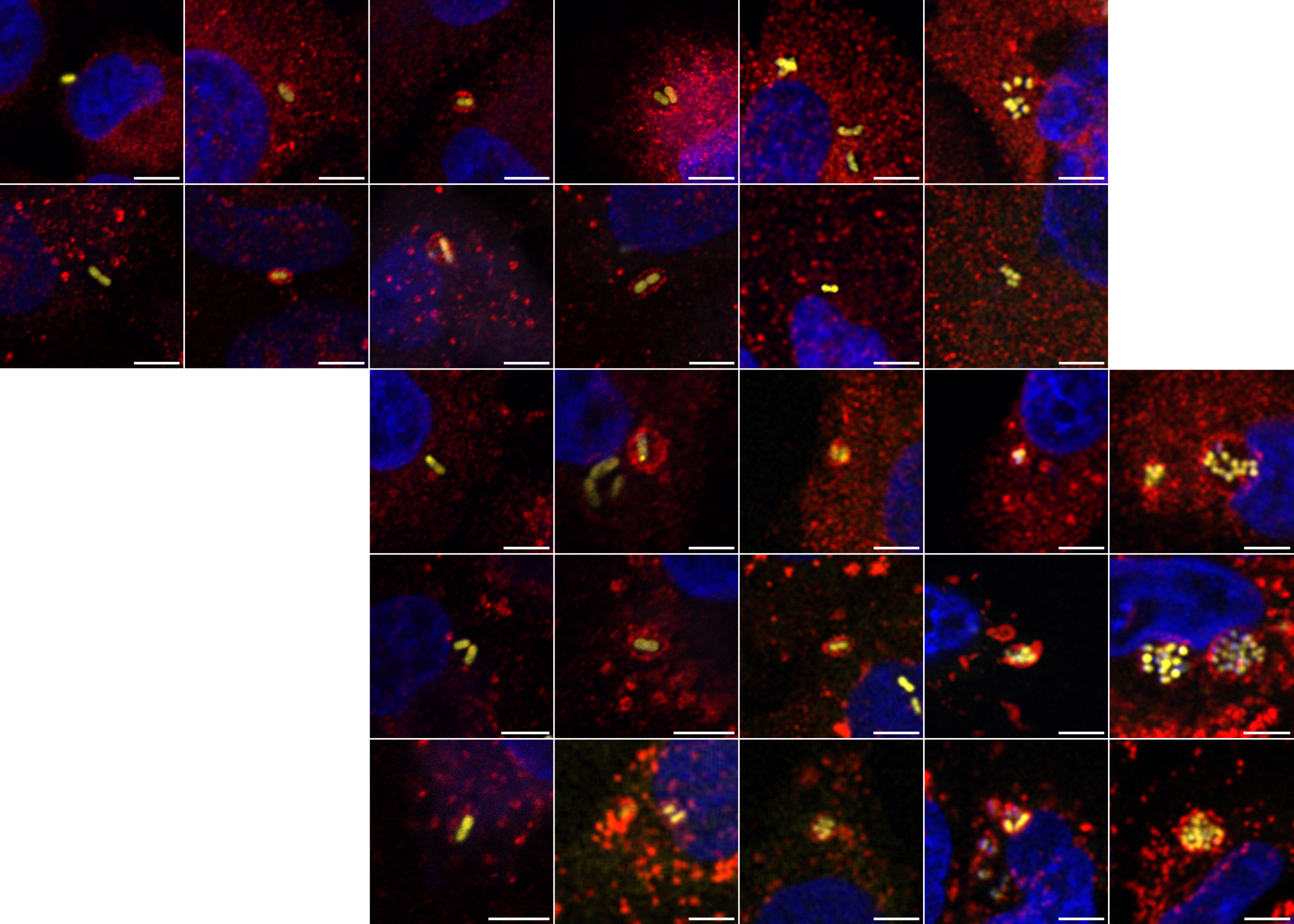
Latest publication: Debruyne et al 2026
Recent studies support that a proportion of clinical Acinetobacter baumannii strains can multiply intracellularly. Here, we defined this transient intracellular niche, which enables efficient bacterial infection of neighboring cells and mapped the bacterial and host gene profiles, highlighting a role for the T2SS in the invasion step. We also demonstrate a remarkable ability for ABC141 to grow in low-oxygen environments.
Just out in PLOS Pathogens!